Spills and Incidents
Phone numbers and contacts
Spills procedures
Reporting an unintentional release of a GMO
Disinfectants - The perfect match for your bug
Security Sensitive Biological Agents- What to do if you suspect the use of an SSBA
To protect each other, our health, our environment, our economy and our research, we must continue to engage in biosafety practices at the highest level.
APPLICATIONS TO THE UNISA INSTITUTIONAL BIOSAFETY COMMITTEE SHOULD NOT BE SUBMITTED IN THE
BCR E-FORM, AS OF 26 SEPTEMBER 2025.
APPLICATIONS TO ADELAIDE UNIVERSITY INSTITUTIONAL BIOSAFETY COMMITTEE WILL BE UNDERTAKEN IN THE ACES E-FORM.
26 September 2025
New applications to UniSA IBC for hazardous biological material and genetically modified organisms, can be submitted using the UniSA IBC Word document. (see below for link to the Word version of the application form.)
7 November 2025
New applications to UniSA IBC must not be started after 7 November 2025, unless urgent.
Please contact biosafety@unisa.edu.au to confirm if your application meets the criteria for an urgent application.
3 December 2025
IBC applications currently lodged in the BCR UniSAfe e-form must either be completed or cancelled by 3 December 2025.
Please review the status of your IBC application in the BCR system. If you are concerned about its progress, please contact biosafety@unisa.edu.au.
5 January 2026
The BCR UniSAfe e-form will not be used at Adelaide University for IBC applications. The Adelaide University e-form ACES will be used and available to UniSA staff for submissions to the Adelaide University IBC from 5 January 2026.
Efforts are being made to give UniSA staff and students access to ACES before 5 January 2026, so that draft IBC applications can be written in ACES.
Details regarding the ACES e-form will be communicated at a later date.
If you are uncertain as to the best approach for your IBC applications, please don’t hesitate to contact biosafety@unisa.edu.au.
Phone numbers and contacts
Spills procedures
Reporting an unintentional release of a GMO
Disinfectants - The perfect match for your bug
Security Sensitive Biological Agents- What to do if you suspect the use of an SSBA
Biological hazardous material is defined as organic substances that pose a threat to the health of humans and other living organisms. Biological hazards include pathogenic microorganisms, viruses, toxins (from biological sources), spores, fungi and bio-active substances. Biological hazards can also be considered to include biological vectors or transmitters of disease. Here is a list of some common sources of biologically hazardous materials.
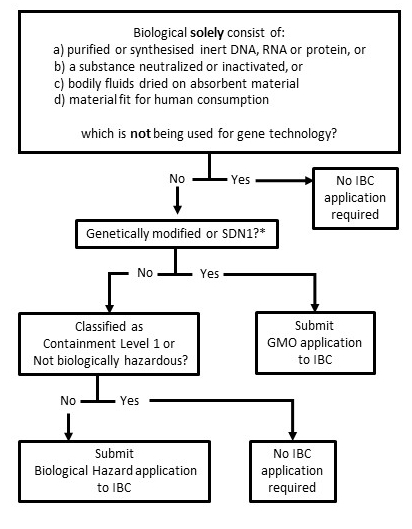
For clarification contact biosafety@unisa.edu.au.
Instititional Biosafety Committee assessment is required before hazardous biological material is used.
Application Forms
Biological Hazard Application Form |
To start a new application select in the left hand panel - Workspaces > BCR Process > BCR Process > +New
Handy HintTo determine the risk rating of a wild-type microorganism or cell line, refer to AS/NZS 2243.3 or the manufacturer's Material Safety Data Sheet. |
Minor Modifications
An amendment application can be submitted to add personnel or facilities to an IBC approved biological process .
If the application was submitted via the online BCR module, changes can be requested via the ‘Change Requests’ tab in the approved BCR application.
If the application was submitted via a Word form, changes can be requested via submission of the
Amendment Application Form |
Send the amendment application form to biosafety@unisa.edu.au before obtaining signature(s) of the Lab Manager/Coordinator(s),
Use of genetically modified organisms requires IBC approval before project commencement. Details and application forms can be found on the
For Biosafety Officer approval to establish an account with ATCC, email biosafety@unisa.edu.au with the:
Before signing, the Biosafety Officer will check that appropriate facilities and risk management strategies are in place.
When working with biologicals, it is important that we don't over estimate our biosafety knowledge and underestimate the risks. Biosafety training is important in avoiding those pitfalls and keeping you and your colleagues safe.
Completion of the online Biosafety 1 training module and laboratory induction are compulsory for all new staff and research students wishing to work with biological material.
It is the responsibility of Research Leaders and Facility Managers to ensure they and the staff and students directly under their supervision, are adequately trained to manage hazardous biological material and GMOs.
Training should include:
UniSA Research Office and the IBC offer training program support. For support and further information contact: biosafety@unisa.edu.au.
Biosafety Information Seminar Recordings
Work involving genetically modified organisms must be conducted in OGTR certified facilities of the same or higher physical containment rating. Exempt GMOs must only be used in OGTR certified facilities of PC1 or higher.
Work involving GMOs must only be conducted within facilities suitable to contain the organism. For example, GM insects can only be used in an OGTR certified PC1 or higher insectary, not in a standard laboratory.
Work involving non-GM organisms of Risk Group 1 or higher grouping, must be conducted in either an IBC classified or OGTR certified biocontainment facility, of equal or higher PC rating.
Whether biological goods are transferred internationally, interstate or just next door there are a number of items to organise. For details see the Biosecurity webpages.
University’s biosafety management system and regulatory requirements for working with infectious microorganisms and genetically modified organisms.
Electronic copies can be accessed through the UniSA Library.
Recommended way to find the Standards:
Important Note: There are no facilities within South Australia currently available to undertake research using SSBAs.
For Biological Hazards applications involving the use of Security Sensitive Biological Agents (SSBA), please check the SSBA website (Department of Health), and SSBA newsletters (published monthly).
National Health Security Regulations 2018
National Health Security Act 2007
Controls are placed on biological material which can be used for military purposes. Export restrictions include not only the export of physical (tangible) goods and technology, but also the supply, publication or brokering of controlled technology by electronic or other non-physical (intangible) means. For further information and a self-assessment tool see our Defence Export Controls webpage.
Staff and Research Degree Students who handle human or animal blood, tissue, excrement or body fluids, soil, unaged compost, pathogenic bacteria, prions, parasites or viruses are often at risk of occupational exposure to vaccine-preventable diseases. Personnel considered at high risk are those in direct physical contact, contact with contaminated surfaces/equipment or exposed to infection by the respiratory route.
Immunisation of Research Staff and Students
To determine if research personnel are at risk of infection of vaccine-preventable diseases, Research Group Leaders will conduct a risk assessment as part of their IBC application to use biologicals. The UniSAfe BCR application form will ask Research Group Leaders specific questions regarding the type of biological material handled, the potential route of infection, and the project procedures.
The type of recommended vaccinations can be found in the Australian Immunisation Handbook. Vaccinations recommended for laboratory workers and people working with blood and body fluids are listed in the 'Vaccination for people at occupational risk' webpage of the AIH.
The IBC will review the application and assess the risks on a case-by-case basis. If the IBC considers the project poses a risk of infection, the IBC will ratify the immunisation recommendations.
Immunisation of Technical and Professional Staff
Vaccination is also available for technical and professional staff at risk of occupational exposure to vaccine preventable diseases.
Line Managers should:
For Everyone – Required Documents:
Staff and Research Degree students requiring vaccination should be given a vaccination form to take to either the UniSA Health Medical Clinic or General Practitioner. For record keeping personnel should return the completed form to the Operations/General Manager of their Academic Unit, Institute or Centre.
Funding
Cost of vaccination can be charged directly to either Academic Unit, Institute, Centre, Portfolio or research project funding. If personnel use the UniSA Health Medical Clinic, a claim can be submitted directly to these funds, avoiding the need for upfront payment. To claim on this system a fs32 form must be completed and submitted to the UniSA Health Medical Clinic. Alternatively, personnel may use their own General Practioner or Travel Doctor and then seek reimbursement through their Research Group Leader or Line Manager.
A schematic representation of processes (600kb) is available. Further information is available through the Biosafety Officer, biosafety@unisa.edu.au.
Members of the Institutional Biosafety Committee register their personal interests which may be perceived by others to conflict with their unbiased assessment of applications. Conflicted members do not read or vote on applications or matters with which they are conflicted.
Applicants are welcome to discuss any concerns about conflicts of interest and request that a specific IBC member not review a matter, by contacting the Executive Officer biosafety@unisa.edu.au.
Have you witnessed a supervisor encouraging unsafe biosafety practises?
Have you seen a colleague engaging in unsafe practices?
Are you concerned that the personal protective equipment is inadequate to provide safety?
Are you concerned about a potential misuse or dangerous modification of a genetically modified organism?
For any of these situations or others of concern, we invite you to contact the Biosafety Officer, biosafety@unisa.edu.au, or the Manager of Research Integrity, researchintegrity@unisa.edu.au.
Your name and personal details will not be revealed to anyone, including the Institutional Biosafety Committee.